Chlorine injection package
Chlorine injection package
Chlorination of refined sewage or chlorination into wells or drinking water is done to eliminate the microbial burden of the water. Due to its high oxidizing properties and ease of access, chlorine is one of the most common methods of disinfection of water or wastewater. The easiest and most cost-effective method of chlorine is to inject chlorine powder (solid) or liquid. Solid chlorine is calcium hypochlorite.

The Abram’s Chloride Injection Package includes the following sections:
- Italian dosing pump
- Clay polyethylene tank
- Liquid storage chassis
- Electromotor and shaft for mixing solid chlorine
- Calcium hypochlorite
Chlorination of sewage
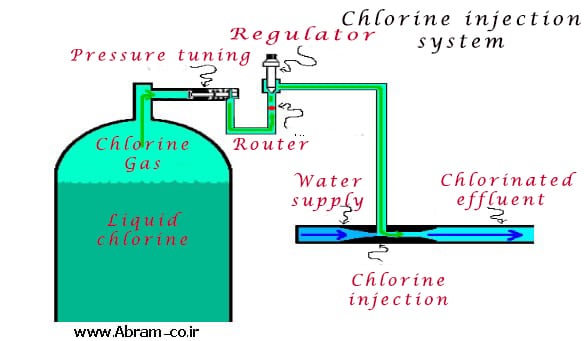
Sewage chlorination is used at the outlet of sewage treatment plants to standardize pathogenic coliforms. One of the methods for disinfection of sewage is chlorine that is suspected of producing trihalomethanes. But considering that the chlorination properties are still most used in disinfection. Drainage Chlorine Drainage Chlorine Design includes three parts of chlorination equipment, chlorine building and chlorine contact tank. The chlorine used in the treatment plant is usually chlorine gas Cl2 or in the form of sodium hypochlorite or calcium hypochlorite. Hypochlorites can be solid or liquid solids in the form of powder or granules.

chlorine The equipment used to inject sodium hypochlorite (liquid) is called hypochlorinator. This equipment consists of a reservoir, which is made up of a high concentration of chlorine solution (10%) called stoic solution in this reservoir. This liquid is then injected into the chlorination pool by the pump.
Simple hypochlorinators, low cost and easy to use. But hypochlorites are expensive compared to gaseous chlorine. Because they have no leakage, their equipment is less risky. So they have a lot of use. Adjust the amount of injected chlorine controlled by a flowmeter and a v-shaped erythesis plate. The V-shaped ophitic plate is used to adjust the gas flow rate.
Problems using chlorine
- If the water contains iron ions, it is manganese, as soon as chlorine is added, they form an insoluble precipitate, and they convert to iron and manganese 3, which precipitates.
- In the presence of organic matter, these substances are consumed during the oxidation process
- The chlorine reaction with ammonia leads to the formation of chloramine, which is known to have a limited disinfection, called chlorine.
In the bottom of the holes, the sputtering efficiency is higher with increasing HOCL, and we also have corrosion in the bottom of the holes, and we have sedimentation in the above holes. In the low ethanol and tertiary chlorine production it is poisonous, so we have to make chlorine and amine produced in the form of monochloroamino in the form of dichloramine. So the best pH is about 7 to 8, which contains about 80% free chlorine in the form of HOCL and 20% in the form of OCL.